Welcome to our ‘Heat Pump Knowledge Series’, a 5-part series on heat pump essentials, with new episodes releasing on every Monday! This series is delivered by Dr.Satyanarayanan Seshadri, Chief Technology Officer at Aspiration Energy, and the Director of Energy and Emissions Lab at IIT Madras. If you have any questions, please let us know in the comments and we will get them answered by Dr.Satyanarayanan.
WELCOME TO THE SERIES!
Fundamentals of thermodynamics
We welcome you to explore this series along with us starting from the fundamentals of Thermodynamics all the way to the complete system design. In Episode 1, we will focus on the basic thermodynamics of a heat pump.
WHY HEAT PUMP?
To understand a heat pump, let’s look at the situation here. We want to move heat from a cold reservoir to a hot reservoir and it is schematically represented here. This is not a spontaneous process. If it does happen spontaneously, it violates Thermodynamics. So, how can this be accomplished? It is typically accomplished by adding external work. And that external work is in the form of a compressor. The electrical energy that is fed into the compressor enables you to move heat from a cold reservoir to a hot reservoir. Just how you would move water from an underground sump to an overhead tank, this moves heat from a low potential zone to a high potential zone.
So, we know that we need to do external work to achieve this benefit. However, we also know that there are no systems where the benefit – the output – is more than the input. And if it does so, it will violate the fundamental second law of thermodynamics.

HOW DOES A HEAT PUMP WORK?
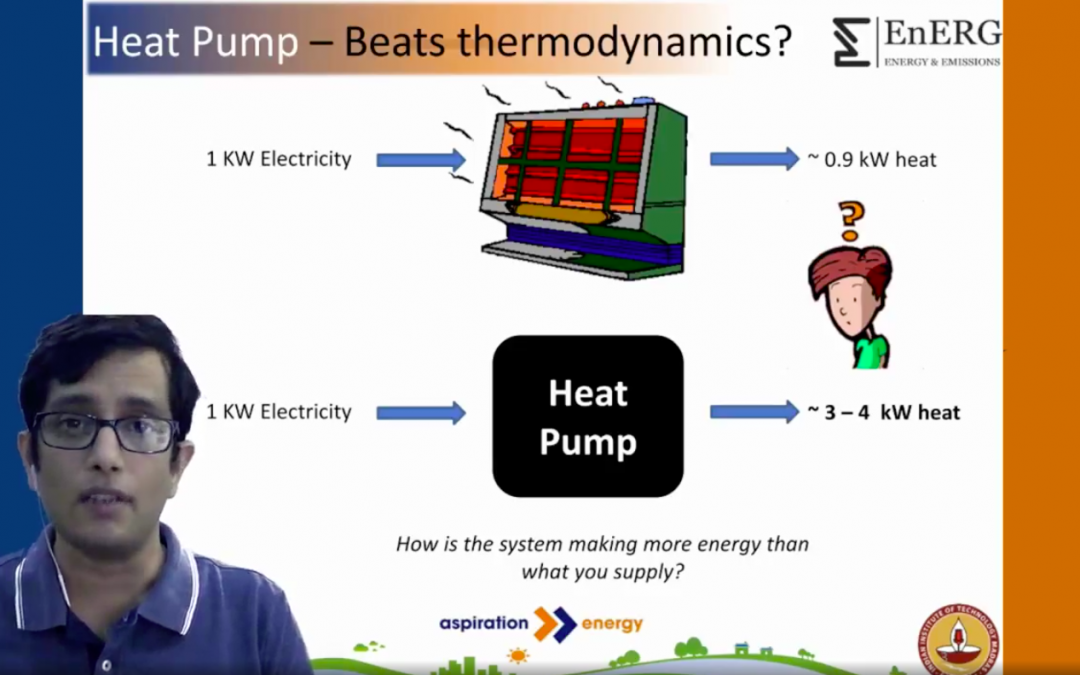
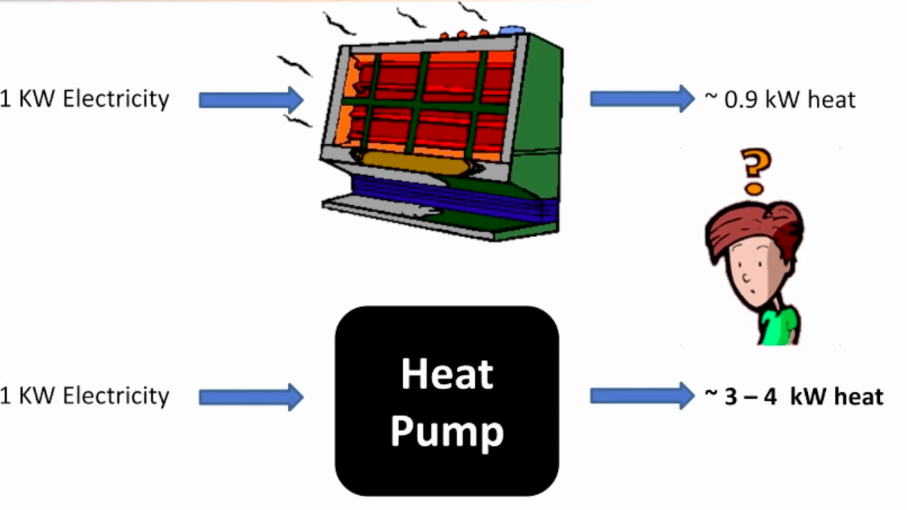
So, how does a heat pump work? We have known people tell us that the heat pump system is capable of achieving efficiencies above 300%… and sometimes even above 700%. How can that be possible? We know that the electrical heater, which we commonly use, takes in about a unit of electricity and produces output that’s worth about only 90%. So, does the heat pump violate the second law? Is it possible that it is a perpetual motion machine? Of course not! The fundamental operating principle of a heat pump is that it is boosting heat which essentially means it is recovering heat from a zone, adding some amount of external energy that is provided and rejecting the heat. So, the sum total energy you put into the heat pump is rejected so there is no violation of the second law. And this is also the reason why heat pump efficiencies can be higher.
COMPARING HEAT PUMP TO A REFRIGERATOR AND AN AC
So, are there systems that you are familiar with that do the same thing? Of course! You have them in your house. You have the refrigeration and the air conditioner both of which do the same job. A refrigerator keeps the space conditioned inside so that all your produce can be fresh. It removes the heat from the cold zone and rejects it to the hot zone i.e outside the refrigerator by utilizing the work input to the compressor. Similarly, the air conditioner keeps the room cool by removing the heat from the conditioned zone and rejecting it in the hot ambient. So, these are two devices that we very commonly utilize and we know that an air conditioner which is a 1 TON system, which is about 3.56 kW, can consume about 0.75 to 1 kW of input power and deliver that kind of output. So, essentially it is working at an efficiency of 350%. How is that possible? It has to do with the way we define the efficiency and it also has to do with a little bit of thermodynamics.
THE THERMODYNAMIC CYCLE EXPLAINED
So, here we have the cycle… the thermodynamic cycle on which a heat pump operates. The first step is generally the compression, where the vapor is taken and the electricity is provided to the compressor to compress it to a high-pressure superheated vapor (1 to 2).
The next step (2 to 3) is to remove the heat in that vapor and then condense it to a high-pressure liquid. So, these two processes actually reject heat to the ambient in case of a refrigerator or an air conditioner and this is the heat that we want to utilize for our process heating in a heat pump.
So, once that heat is removed and the vapor becomes a completely saturated liquid (3 to 4), the next process is called expansion where you throttle from high pressure to low pressure (4 to 5) and in the process, the temperature decreases and point 5 in the figure are tuned so that you can remove heat from whichever conditioned environment. For example, if you want to remove heat from a refrigerator, it is tuned in such a way that it maintains the temperature of a refrigerator. For an air conditioner, say, it is tuned to such that you can maintain 15 or 16 degrees in an air-conditioned room. So, that is depending on the application and once it is tuned there, it absorbs heat from conditioned space or from the ambient and it comes to saturated vapor, the cycle continues again.

HOW TO EXPRESS EFFICIENCY AND WHAT’S A COP?
So now, how do we express efficiency? We generally express efficiencies by the useful work done (divided) by the energy supplied or the input provided. In this case for us, the useful work is the heat that we received from the condenser. So, (efficiency is) the heat delivered by the condenser to the work input to the compressor. This combines and gives us total efficiency. And now you can see why the efficiency can be beyond a hundred because heat delivered by the condenser combines all the energy input to the system including the ambient heat. So, we also talked about heat pumps and air conditioners being related. How is it related? COP of an air conditioner is basically heat absorbed by the evaporator divided by the work input to the compressor which is nothing but useful work for us by the air conditioner is how much cooling that it does to a room that is useful work and to do that how much energy needs to be supplied to the compressor and they are related by a simple arithmetic expression that you see here. So, this equation is true for vapor compression heat pumps. Of course, there are many other types of heat pumps such as thermochemical, which is absorption, adsorption systems and thermo compressors. They all work on slightly different thermodynamics. Nevertheless, the fundamental principle of a heat pump is that it extracts heat from a low-temperature source and rejects it to a high-temperature source and in the process, depending on the nature of the temperature sources, the efficiency can be much higher than what you expect.
WHAT’S COMING UP NEXT?
Now that you know how a heat pump system works, the next things that you really need to know are
- what are the components of a heat pump system?
- how are they designed and selected?
- how does that affect the performance of the system that you decide to implement?
And for that, stay tuned, and let’s meet again in the second episode. Thanks for joining!